
COVID RASH VACCINE CODE
The virus' genetic code is delivered to the leaf cells of the plant using harmless bacteria.

This type of vaccine uses plant-based technology. Learn more about protein subunit vaccines Plant-based vaccines They can't cause COVID-19 because they only contain small purified pieces of proteins and not the virus itself. Protein subunit vaccines are already used to protect against other diseases, such as hepatitis B. Once vaccinated, your immune system triggers an immune response that will help protect you against COVID-19.

Protein subunit vaccines contain harmless and purified pieces (proteins) of the virus, which have been specifically selected for their ability to trigger immunity. Learn more about viral vector vaccines Protein subunit vaccines The adenovirus isn't the virus that causes COVID-19. The protein will trigger an immune response that will help protect you against COVID-19. This delivery system helps your cells to make a coronavirus protein. This type of vaccine uses a harmless virus (in this case, an adenovirus) as a delivery system. Learn more about mRNA vaccines Viral vector vaccines You can't get COVID-19 from the vaccine and it doesn't interact with your DNA or change it in any way. MRNA vaccines don't use a live virus to trigger an immune response. These antibodies help you fight the infection if the real virus does enter your body in the future. Once triggered, your body makes antibodies. This protein will trigger an immune response that will help protect you against COVID-19. This type of vaccine helps your cells make a coronavirus protein. Learn more about vaccine safety in Canada mRNA vaccines The vaccines can't give you COVID-19 because they don't contain the virus that causes it. The benefits of all COVID-19 vaccines continue to outweigh the risks of the disease. COVID-19 vaccines are tested during their development according to international standards and then carefully reviewed by Health Canada. Only vaccines that meet the safety, effectiveness and quality standards of Health Canada are approved for use in Canada.
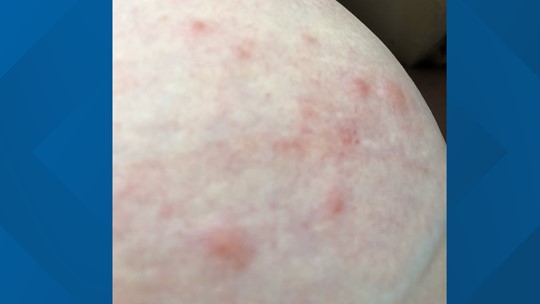
Ongoing monitoring of side effects and reactions. an increase in specific reports), initiates an investigation into a possible correlation with the vaccine.įurther information on reports of suspected adverse reactions to the authorised vaccines can be found on the Swissmedic website. The Swissmedic reporting office assesses any reports and, if concerning patterns emerge (e.g. In Switzerland, any severe side effects must be reported to the authorities. The safety and the efficacy of vaccines are constantly monitored, both within Switzerland and worldwide. You will find further information on th ese and other very rare serious side effects on the website. These cases have been serious, but have occurred in only a very small number of instances (one to eight in every million vaccinations) within the first three weeks after a vaccination with the Janssen viral vector vaccine. These have included rare forms of thrombosis combined with a low blood platelet count ( thrombosis with thrombocytopenia syndrome, or TTS). In very rare cases, severe side effects have been experienced following vaccination with the Janssen viral vector vaccine since this was approved. If you have experience d inflammation of the heart muscle or the pericardium after vaccination, you should discuss with your doctor how best to proceed. The risk of inflammation of the heart muscle or the pericardium is actually greater after infection with the coronavirus than it is following an mRNA vaccination. Consult your doctor immediately if you experience such symptoms. Typical symptoms of heart muscle inflammation are chest pain, shortness of breath, fatigue and palpitations. The majority of these cases were mild and could be treated effectively. In very rare cases, inflammation of the heart muscle or the pericardium has been observed shortly after vaccination (usually within 14 days). Such a reaction usually occurs immediately after the vaccination and is easy to treat. In individuals with a history of severe allergic reactions, appropriate precautionary measures must be observed in the event of a vaccination. In very rare cases, severe side effects may occur, such as an allergic reaction. General symptoms such as shivering, feeling feverish or temperatureĬases of nettle rash have been reported after a booster vaccination. Reaction at the injection site such as pain, redness and swelling. They are usually mild and of short duration. As is the case with all medications, vaccines can cause side effects. The vaccines administered in Switzerland are safe and effective.







 0 kommentar(er)
0 kommentar(er)
